Abstract
Internal tandem duplication mutations in the Fms-like tyrosine kinase 3 (FLT3-ITD) are frequently recurring in AML and confer a poor prognosis. FLT3 inhibitors (FLT3i) such as gilteritinib are efficacious in relapsed AML. Clinical responses to FLT3i include myeloid differentiation of the FLT3-ITD clone in about 50% of patients. How FLT3i induce this response in a subset of patients is unknown.
The FLT3i-induced differentiation response seen in clinical trials has not previously been demonstrated in animal models. We modeled FLT3i-induced differentiation in murine Flt3 ITD/ITDDnmt3a -/- AML model (Meyer et al., Cancer Discovery, 2016). Treatment with FLT3i in vitro accelerated differentiation of cKIT+ leukemic splenocytes as assessed by colony morphology in serial re-plating assays. To characterize the differentiation response in vivo, we transplanted CD45.2+ leukemic splenocytes from moribund mice into sub-lethally irradiated healthy congenic CD45.1+ mice. After confirmation of engraftment at 2 weeks post-irradiation, mice were treated with vehicle or gilteritinib for 4 weeks. Animals treated with gilteritinib demonstrated increased neutrophil and decreased stem/progenitor cell populations, recapitulating the clinically observed increase in granulocytic differentiation of the FLT3-ITD clone.
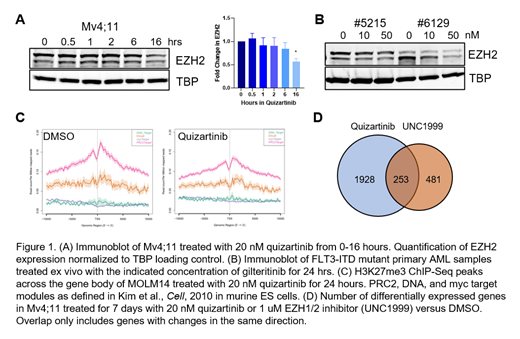
We next sought to understand the molecular mechanism of FLT3i-induced differentiation. We used a proteomic-based screen in a human AML cell line treated with FLT3i to identify novel targets of FLT3-ITD that could be potential mediators of the differentiation response. We identified downregulation of Enhancer of Zeste Homolog 2 (EZH2), the catalytic component of the Polycomb Repressive Complex 2 (PRC2). EZH2 and PRC2 were previously shown to be required for leukemic maintenance in mouse models of MLL-AF9 AML. We treated murine Flt3 ITD/ITDDnmt3a -/- cKIT+ leukemic splenocytes with FLT3i or the EZH1/2 inhibitor (EZH1/2i). Both promoted myeloid differentiation to similar degrees as assessed by colony morphology in this model. We hypothesized that FLT3-ITD regulates EZH2 to maintain leukemia cells in a stem/progenitor cell state. We, therefore, characterized the effect of FLT3i on PRC2 in more detail. We confirmed that FLT3i decreases EZH2 protein levels in FLT3-ITD cell lines and primary human AML within 24 hours of treatment as suggested by our proteomic data (Figure 1A-B). We found that the mechanism of EZH2 downregulation is complex with both transcriptional effects and a decrease in EZH2 protein half-life. ChIP-Seq for H3K27me3 demonstrated decreased peaks at the transcription start sites of PRC2 target genes (Figure 1C). RNA-Seq gene expression profiles of FLT3i- and EZH1/2i-treated human AML cells overlapped at 253 differentially expressed genes (Figure 1D). Critically, both FLT3i and EZH1/2i expression profiles enriched in differentiated myeloid cell gene signatures.
Overall, we found that EZH2 is a novel, unexpected, and clinically relevant target of FLT3-ITD. Our data suggest that reduced EZH2 activity following FLT3 inhibition promotes myeloid differentiation of FLT3-ITD leukemic cells, providing a mechanistic explanation for the FLT3i-induced differentiation response seen in patients. These data demonstrate that FLT3-ITD has at least two functions in leukemogenesis, the well described activation of signaling pathways, and second, a previously undefined, regulation of PRC2 to maintain a myeloid stem cell state. Our results may lead to improved approaches to therapy for FLT3 mutated AML.
Bernt: Syndax: Research Funding; Merck: Other: Spouse is an employee of Merck.. Carroll: Incyte Pharmaceuticals: Research Funding; Janssen Pharmaceutical: Consultancy.